Discussion on galenics of recommended therapies
Actinic keratoses (AK), a precursor of squamous cell carcinoma, are caused by the cumulative UV exposure, preferably UVB, from natural sunlight and/or tanning beds. Your Incidence increased significantly in the last decade. The causes are increasing life expectancy combined with occupational and private UV exposure. According to estimates, in Germany alone 1.7 million patients treated with AK.
The number of unreported cases of people suffering from AK cannot be determined. Evidence-based recommendations for the therapy of AK are being made in the S3 Guideline Actinic keratoses and squamous cell carcinomas of the skin the Association of the Scientific Medical Societies (AWMF) listed. Effects of the treatment methods on the environment are not taken into account.
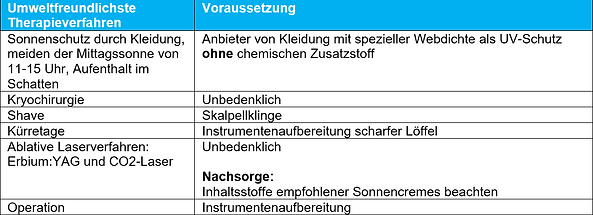
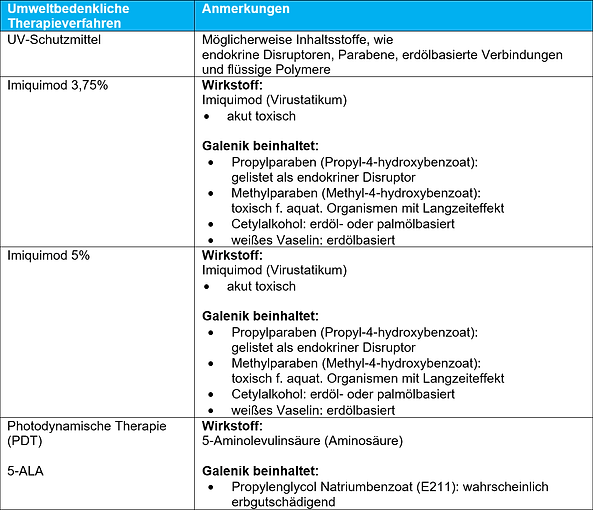
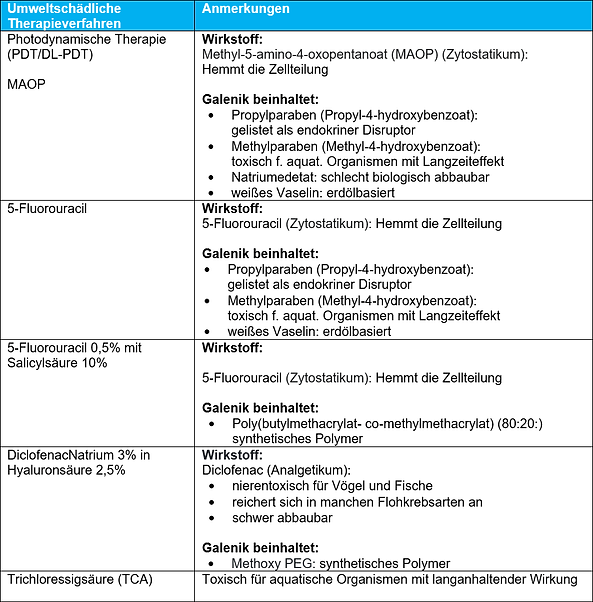
The following tables discuss the environmental friendliness of the galenic composition and excipients used in some of the therapeutic options for AK presented in the AWMF guideline. Direct health effects due to ingredients such as skin irritation or allergies are not named. Also, energy and water consumption through production of the externals as well as greenhouse gas emissions resulting e.g. from production and supply chains are not listed.
Conclusion:
- Numerous externals contain questionable ingredients that can accumulate in the environment and cause lasting damage.
- Some active substances such as cytostatics, TCA and diclofenac are subject to separate disposal, as they are not allowed to enter the waste water.
- The proper disposal of medicines with toxic ingredients by patients and practices is regulated by the Medicinal Products Disposal Act (AMG) not sufficiently ensured.
- It shows that similar treatment methods should also be evaluated on the basis of their environmental friendliness in order to make therapy decisions for patients.
- It is recommended that in future medicinal externals be checked for ingredients of concern so that the findings can be included in the AWMF guidelines.
The prompt interdisciplinary identification of further therapeutics that are available worldwide without prescription but have toxic environmental effects and thus on human health would be desirable. For example, over-the-counter medicines such as diclofenac and also peeling substances such as TCA should be reserved exclusively for medical practice.
Since 01.09.2021, with Tirbanibulin a new active substance on the market that could not yet be considered in the AWMF guideline. It is a microtubule and Src tyrosine kinase inhibitor and is thus a cytostatic. It is therefore to be classified as an environmentally harmful therapy method. Its galenics are harmless to the environment.
Appendix
The chemical ingredients and references listed in the table are provided as a link below:
© Dr. med. Dipl. Biol. Susanne Saha and Dr. med. Christina Hecker 10/2021







